High-Potency Super-Cocaines: The Nightmare Yet To Come

You've heard of Fentanyl, Cathinone "legal highs", and artificial Cannabinoids. What you may not have heard of, are the ultra-powerful artificial cocaines. The first two are over 1000 times more powerful, respectively. There's never been a list of these, until now.
Whatever the legality and morality of central nervous stimulants, cocaine itself - a phenyltropane - is a stunningly beautiful molecule. As are many others in the same family. But there is some utterly enchanting about this little thing, even if the shrub itself is ugly.
A Primer On Cocaine
Cocaine (Erytroxylin) is a tropane alkaloid that is extracted from the leaves of the Coca shrub (Erythroxylum coca), which is native to South America. The biosynthesis of cocaine in the coca plant is regulated by various environmental factors, such as light, temperature, and nutrient availability. The process is also influenced by the developmental stage of the plant, with young leaves typically containing higher concentrations of cocaine than mature leaves.
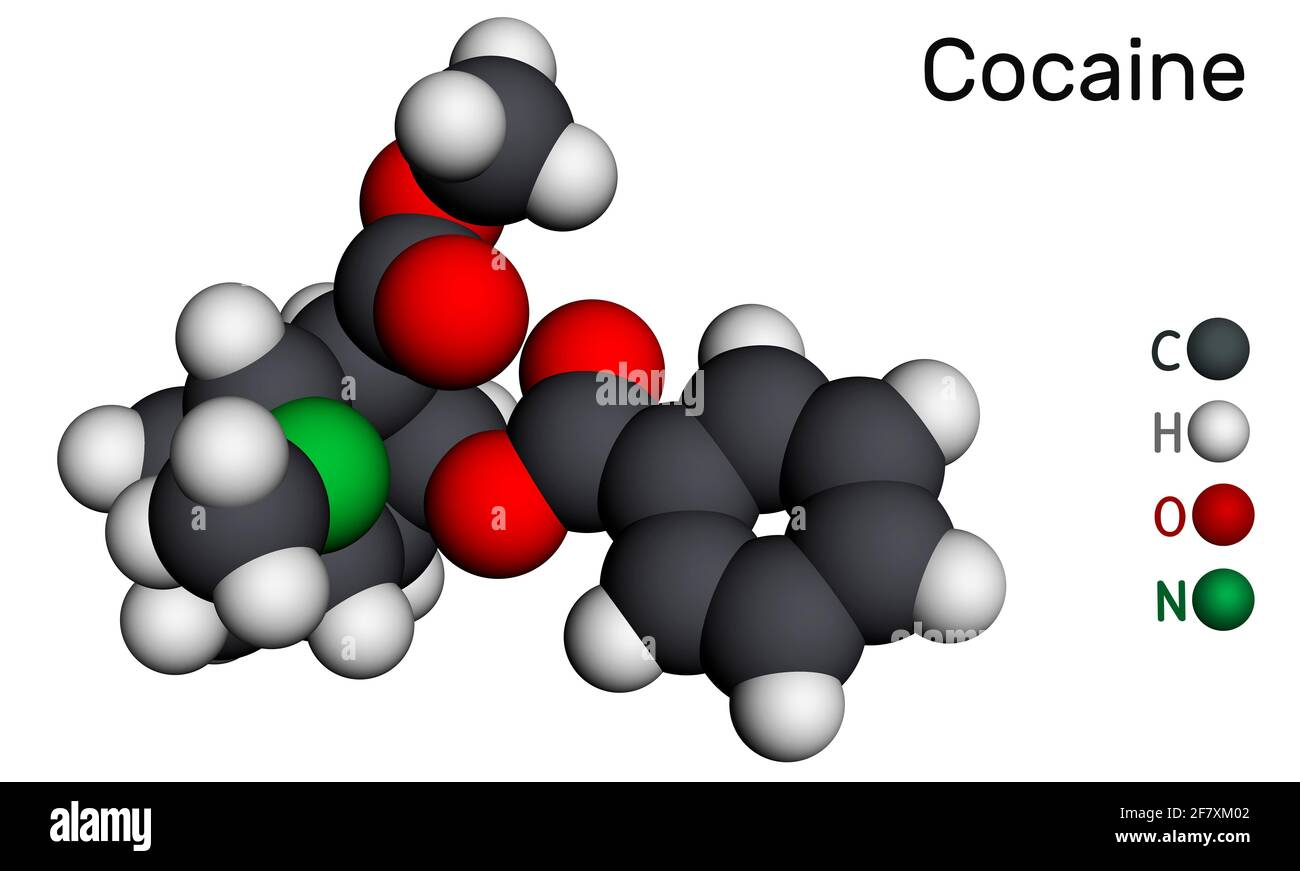
It is a scientific marvel, and a scientific mystery.
The main theories of why it synthesizes cocaine are that it may be a) a defense mechanism to protect itself from herbivorous insects and animals (deer etc), b) it could inhibit the growth and development of competing plant species in the vicinity, c) it's the plant's stress response, and d) an evolutionary adaption.
Cocaine typically makes up 0.5-1.5% of the leaf's dry weight. However, some studies have reported concentrations as high as 2.5% in certain coca varieties. Cis-cinnamoylcocaine (cis-CCC) is the second most abundant (0.1-0.8%), above its trans isomer Trans-cinnamoylcocaine (0.02-0.2%). Benzoylecgonine (0.1-0.4%), Ecgonine (<0.1%), Tropine (< 0.01%), and Hygrine (0.1-0.2%) make up the rest.
A typical dose is 10-120 mg, with 1.2 grams being the estimated lethal dose. Blood concentrations of 0.2-0.5 mg/L are considered toxic. Overdose causes agitation, tremors, hyperthermia, tachycardia, hypertension, cardiac arrhythmias, seizures, and respiratory failure. It can be detected with Cobalt(II) thiocyanate, where it changes pink to blue.
Its various chemical names are:
- Benzoylmethylecgonine
- Erytroxylin
- methyl (1R,2R,3S,5S)-3-benzoyloxy-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate
- 2-beta-Carbomethoxy-3-beta-benzoxytropane
- 3-Tropanylbenzoate-2-carboxylic acid methyl ester
- (1R,2R,3S,5S)-2-Methoxycarbonyltropan-3-yl benzoate
Its identifiers are:
- C17H21NO4
- CN1C2CCC1C(C(C2)OC(=O)C3=CC=CC=C3)C(=O)OC
Chemically-speaking, it is quite similar to the antimuscarinic alkaloid Hyoscyamine, found in the Nightshade family.
Ref: https://pubchem.ncbi.nlm.nih.gov/compound/Cocaine
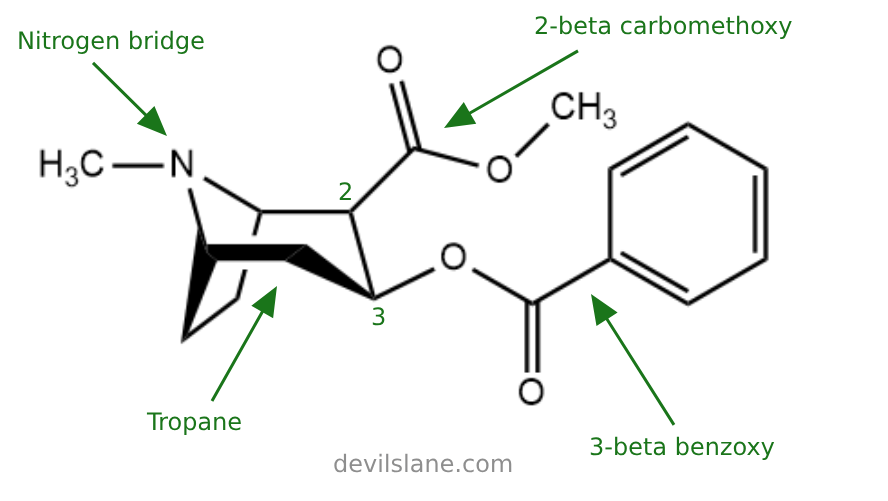
Its core structure includes a tropane ring system composed of a cycloheptane ring fused to a pyrrolidine ring. Key functional groups include a benzoyl ester, a carbomethoxy group, and a tertiary amine. The presence of the tropane ring and aromatic benzoyl ester are key to its stimulant and reinforcing effects.
It acts primarily by blocking the reuptake of neurotransmitters serotonin, norepinephrine, and dopamine. This leads to increased concentrations of these neurotransmitters in the synaptic cleft, enhancing their effects.
The blockage of dopamine reuptake, in particular, is thought to be a key mechanism behind its highly reinforcing and addictive properties. Dopamine is part of the brain's reward pathway, and the increased dopamine signaling triggered by cocaine leads to short-term euphoria as well as changes in the brain that can lead to long-term addiction with repeated use.
The alkaloid is biosynthesized in the leaves of the shrub through a complex series of biochemical reactions involving amino acids, vitamins, and other precursor molecules:
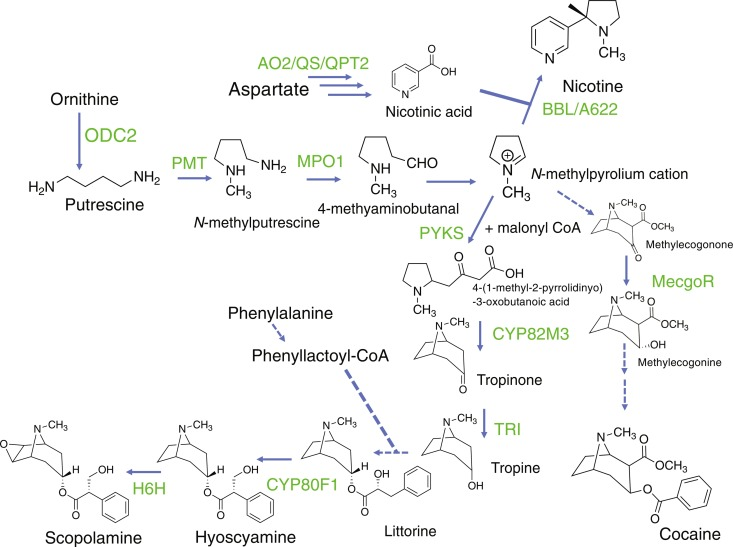
- Amino acid precursors: The biosynthesis starts with the amino acids ornithine and arginine, which are derived from glutamine. These amino acids provide the nitrogen atom necessary for the tropane ring structure.
- Formation of putrescine: Ornithine undergoes decarboxylation by the enzyme ornithine decarboxylase to form putrescine, a diamine compound.
- Methylation to form N-methylputrescine: Putrescine undergoes methylation by the enzyme putrescine N-methyltransferase, with S-adenosyl methionine (SAM) acting as the methyl donor, to form N-methylputrescine.
- Oxidative deamination to 4-methylaminobutanal: N-methylputrescine undergoes oxidative deamination by the enzyme diamine oxidase to form 4-methylaminobutanal.
- Cyclization to form the tropane ring: 4-methylaminobutanal undergoes spontaneous cyclization to form the tropane ring structure, resulting in the formation of the molecule hygrine.
- Addition of acetyl-CoA: Hygrine undergoes condensation with acetyl-CoA, catalyzed by the enzyme hygrine acyltransferase, to form acetylhygrine.
- Benzoylation to form cocaine: In the final step, acetylhygrine is converted to cocaine through benzoylation. This reaction is catalyzed by the enzyme cocaine synthase, which transfers a benzoyl group from benzoyl-CoA to the tropane nitrogen atom.
Ornithine --> Putrescine --> N-methylputrescine --> 4-methylaminobutanal --> hygrine --> acetylhygrine --> Cocaine.
4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoic acid (MPOA) is converted into Cocaine by the EnCYP81AN15 and EnMT4 enzymes. It's notable MPOA is converted to methyl 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoate (MMPO) to produce Hyoscyamine.
More: https://en.wikipedia.org/wiki/Biosynthesis_of_cocaine
What is a Phenyltropane?
Tropanes are beautiful. Atropine, another one, has saved countless lives as an antidote to nerve gases.
Let's break the cocaine molecule down into its constituent parts to learn a little more. Here are the elements of cocaine's structure:
- 2-beta-carbomethoxy (an ester)
- (a benzene-oxygen)
- tropane (the base molecule)
The structure occurs in a pattern:
Tropane Ring
Tropane alkaloids occur in plants of the families Erythroxylaceae (including coca) and Solanaceae (including mandrake, henbane, deadly nightshade, datura, potato, tomato).
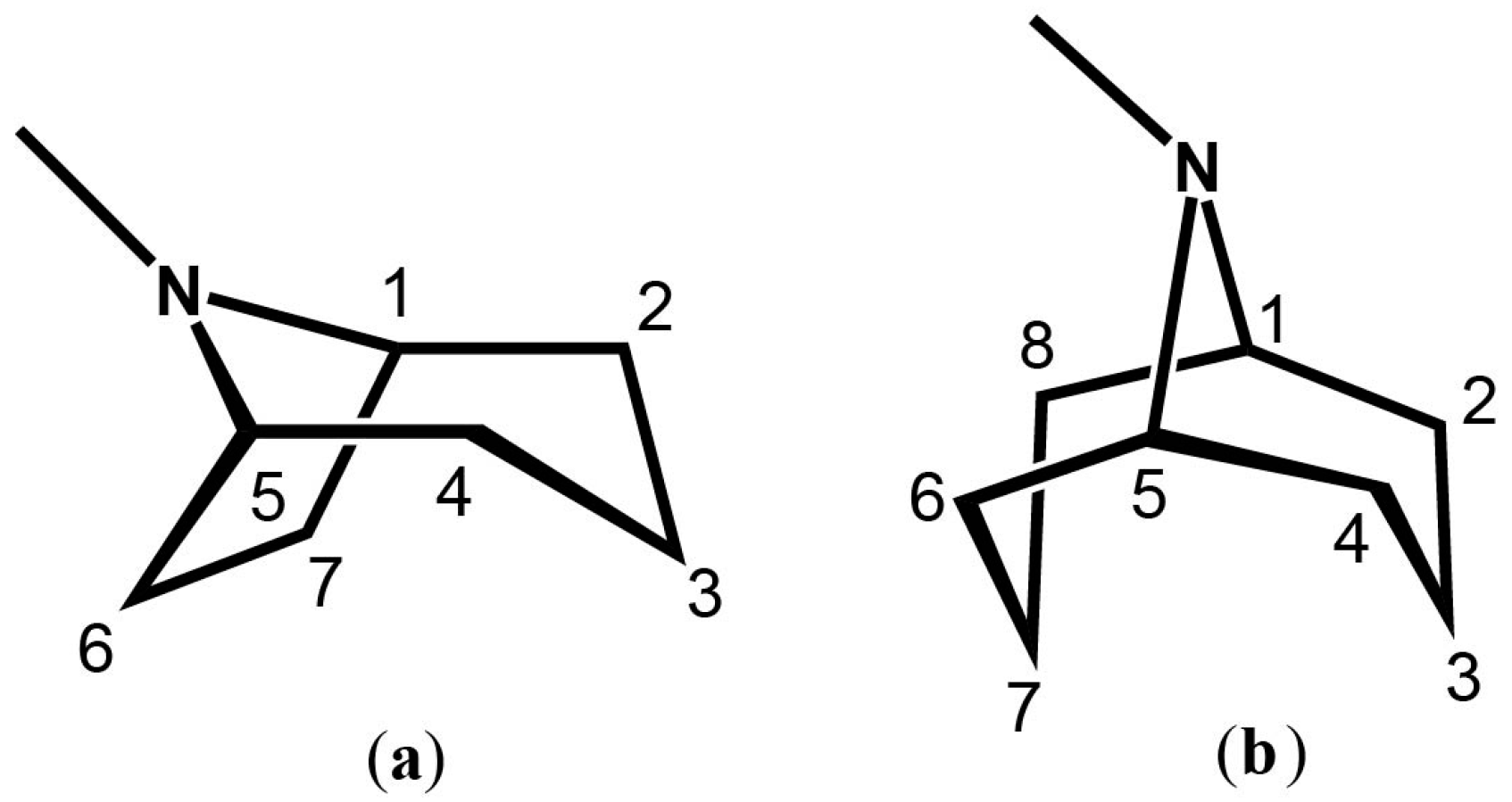
It's a cycloheptane (7 sides, technically "bicyclic ", made of 2 things), with a nitrogen bridge (top, in between carbons 1 and 5) with an additional methyl group attached to the it. What a weird looking thing.
The skeleton consists of two fused rings: a pyrrolidine ring and a piperidine ring. The pyrrolidine ring is a 5-membered ring containing one nitrogen atom, while the piperidine ring is a 6-membered ring also containing one nitrogen atom. These two rings share the nitrogen atom and two carbon atoms.
- The numbers represent the position of each carbon atom in the tropane skeleton.
- The shared nitrogen atom is at position 1.
- Positions 2 and 3 are part of the pyrrolidine ring.
- Positions 4, 5, 6, and 7 are part of the piperidine ring.
- Position 8 is the carbon connecting the two rings, known as the bridgehead carbon.
The stereochemistry of tropane alkaloids is important for their biological activity. The bridgehead carbon (C-8) is a chiral center, meaning it can have two different configurations. The orientation of the substituents at this position can greatly influence the compound's pharmacological properties.
Tropane alkaloids often have various substituents attached to the tropane skeleton, such as hydroxyl (-OH), acetoxy (-OC(O)CH₃), or phenyl (C₆H₅) groups. These substituents can be attached at different positions, most commonly at the nitrogen atom (N1) or the carbons adjacent to it (C2, C3, C6, or C7). The type and position of these substituents contribute to the diverse range of biological activities exhibited by tropane alkaloids.
3-beta-benzoxy
A 3-beta-benzoxy group is a benzene ring attached to an oxygen atom at the 3-beta position of the base molecule (in this case, the tropane).
- "3" refers to the position of the substitution on the main ring system, which is typically numbered.
- "Beta" (β) indicates the stereochemical configuration at the 3-position. In steroid chemistry, "beta" means the functional group is oriented above the plane of the main ring system.
- "Benzoxy" is derived from "benzene" and "oxy." It indicates that a benzene ring (C₆H₅) is connected to the main molecule via an oxygen atom (-O-).
2-beta-Carbomethoxy
A 2-beta-carbomethoxy group is a methyl ester attached to the 2-beta position of the base molecule (the tropane).
- "2" refers to the position of the substitution on the main ring system, which is typically numbered.
- "Beta" (β) indicates the stereochemical configuration at the 2-position. In the context of tropane alkaloids, "beta" means the functional group is oriented above the plane of the main ring system.
- "Carbomethoxy" is a combination of "carbo-" (referring to the carbonyl group, C=O) and "methoxy" (referring to the methyl ester, -OCH₃). It indicates that a methyl ester group is attached to the main molecule.
A methyl ester has the general structure: R-COO-CH₃, where R represents the rest of the molecule.
Can It Be Made In A Lab?
Of course. But growing it is much easier. The plant is a lab. But there are key differences to producing a compound as complex as Cocaine, in comparison, to, say, amphetamines (which involves basically taking an oxygen off Phenylacetone with aluminium isopropoxide so it becomes 1-phenyl-2-propanol, or reducing Ephedrine with ammonia over lithium so it becomes the equivalent 4-methylated 1-phenyl-propan-2-amine).
The total chemical synthesis of cocaine involves a longer series of reactions and transformations: a tropane ring system, a benzoyl ester, and a carbomethoxy group. It's not trivial. The precursors required, such as ecgonine or its derivatives, are not as readily available. Cocaine has several chiral centers, meaning that different stereoisomers of the molecule can exist. Producing the desired stereoisomer requires careful control over the reaction conditions and may involve additional purification steps, which adds to the complexity of the synthesis.
The first total synthesis of cocaine was achieved by Richard Martin Willstätter in 1901 (other sources say 1896 or something, wrong) in a 17-stage process where he prepared the tropane ring system with a double bond dibromination to prime a cyclisation reaction.
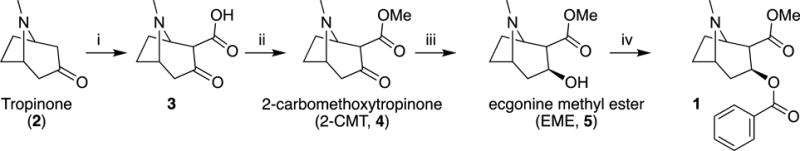
In the mid-1900’s, other syntheses of (-)cocaine emerged utilizing another natural product, l-ecgonine, and achieving 95% conversion after esterification and benzoylation. Most total syntheses of cocaine, regardless of starting material, included 2-carbomethoxytropinone (2-CMT) and focused effort on optimizing the steps to this intermediate.
In 1974, generation of tropane alkaloids was facilitated by use of the polybromo ketone-iron carbonyl reaction of 1,3-dibromopropan-2-one with methyl 1H-pyrrole-1-carboxylate to access complex intermediates such as tropinones and tropine. Another interesting route involved [4+2] nitroso cycloadditions of 1,3-cycloheptadienes and 1-chloro-1-nitrosocyclohexane via a Diels-Alder reaction. However, these attempts were unable to control the stereochemistry of cocaine.
The exact structures and physical properties of four of the eight possible diastereomers (cocaine, pseudococaine, allococaine and pseudoallococaine) were described, and the focus shifted toward controlling the stereochemistry in the synthesis. Optimized total syntheses of cocaine quickly followed the advent of nitrone cycloaddition. For example, Tufariello started from 3,4-dihydro-2H-pyrrole 1-oxide. A cycloaddition with methyl but-3-enoate gave nitrone 25, which was converted to [26] with mCPBA. This was then converted to isoxazolidine ester which, upon refluxing in xylene, rearranged to give the key cycloadduct; from this intermediate they were able to control the stereochemistry and make (-)-cocaine in three additional steps.
A recent total synthesis of (-)-cocaine by Cheng et al., was accomplished in nine steps beginning with Betti base derivative. After five steps, including a Grubbs II catalyzed ring closing metathesis and a 1,3-dipolar cycloaddition, the 3-bromo-2-isoxazoline intermediate was synthesized. After four more steps to open the isoxazoline ring, install the benzoyl substituent in a stereoselective fashion, and deprotect the amine, (-)-cocaine was produced in 55% overall yield. Shing & So also developed a 15 step synthesis in 2011 (13% overall yield), starting from the cheap and commercially available D-(-)-ribose, which allowed for analogue derivation at the C6 and C7 positions through a key endo-selective intramolecular nitrone-alkene cycloaddition reaction. An alternative synthesis, which focuses on synthetically simple techniques, controls stereoconfiguration and permits access to many active tropane alkaloids. They begin with a one-pot catalytic, enantioselective three-component reaction catalytic aza-Michael/Witting tandem reaction between enal, hydroxylamine, and alkyl 2-triphenylphosporanylidene acetate, to yield the ester. The synthesis to active cocaine progressed in 5 total steps and 39% overall yield.
DARK Classics in Chemical Neuroscience: Cocaine
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6197930/
For a comprehensive read on many of the different methods:
- https://synarchive.com/syn/303
- https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-0039-1690529.pdf
- https://onlinelibrary.wiley.com/doi/abs/10.1002/jhet.5570240104
- https://www.researchgate.net/publication/258111122_A_Practical_Total_Synthesis_of_Cocaine's_Enantiomers
- https://pubs.acs.org/doi/10.1021/ol048777a
- https://pubs.acs.org/doi/10.1021/jo200069m
It's not impossible, but it's tricky, labour-intensive, and expensive.
The Big, Bad Ugly List Of Super Analogs
It's difficult to rank these because so little research has been done. To the author's knowledge, there is no list of these anywhere. Not a BIG list anyway.
Most of the compounds involve simple substitutions of groups onto the tropane to make it "stickier" in the brain, so to speak.
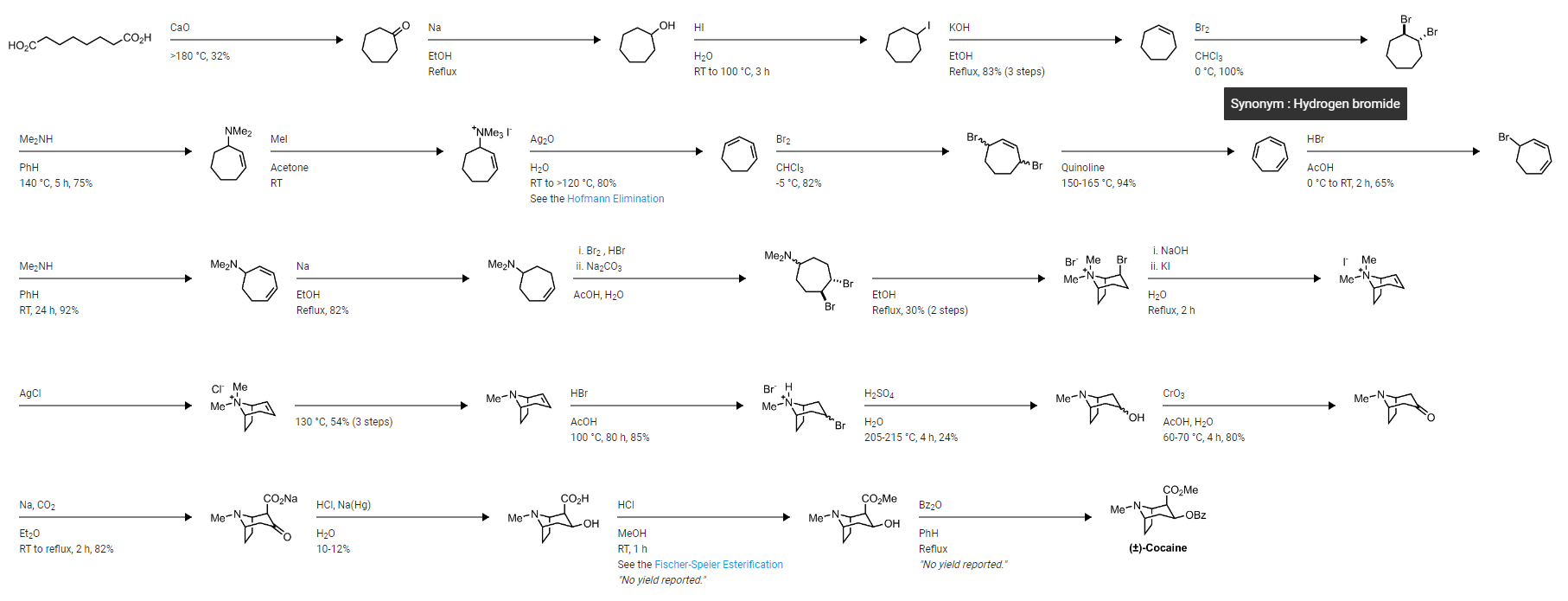
To understand potency, we need to understand half maximal inhibitory concentration, or IC50. It is a measure of the potency of a substance in inhibiting a specific biological or biochemical function by half, i.e. it's half-life, so to speak. In pharmacology, IC50 quantifies the concentration of a drug or inhibitor required to inhibit a biological process, such as an enzyme reaction, cellular function, or cell proliferation, by 50%.
IC50 values are typically expressed in molar concentration units, such as micromolar (μM) or nanomolar (nM). A lower IC50 means a compound is more potent because it requires less of the substance to achieve the same level of inhibition of the transporter in question. The formula to calculate the potency multiplier is:
Potency Multiplier = IC50 of Analog / IC50 of Cocaine
Example: The IC50 of cocaine is given as 173 nM in a study. Analog 13 (HD-23, P2-NT, 2-Naphthyl COCH2CH3 H) with IC50 is 0.20 nM.
Potency Factor= 0.20 / 173
Result: To achieve the same effect as cocaine, which has an IC50 of 173 nM, you would need only 1/865th (0.116%) the amount of Analog 13 (HD-23, P2-NT, 2-Naphthyl COCH2CH3 H), as its IC50 is 0.20 nM. This means HD-23 is approximately 865 times more potent than cocaine.
Acronyms:
- WF: Wake Forest (University)
- WIN: Sterling-WINthrop Research Institute
- RTI: Research Triangle Institute
- HD: Huw Davies
The Pharmacological View
A standard dose of cocaine is estimated to be 100mg for the purposes of this chart.
| Name | Potency | Dose |
|---|---|---|
| WF-23-2 | 1000-5400x * | 0.02-0.1 mg |
| WF-23-1 | 865-1200x | 0.08-0.12 mg |
| RTI-55 | 100x | 1 mg |
| RT1-121 | 150x | 0.67 mg |
| RTI-31 | 81x | 1.23 mg |
| WF-24 | 68x | 1.47 mg |
| RTI-51 | 52x | 1.92 mg |
| RTI-32 | 52x | 1.92 mg |
| WF-30 | 32x | 3.13 mg |
| WF-11/2-PTT | 20x | 5 mg |
| HD-4/WF-4 | 17.65x | 5.67 mg |
| HD-12/WF-8 | 17.13x | 5.84 mg |
| RTI-112 | 5x | 20 mg |
| WIN-35428 | 10x | 10 mg |
| RTI-177 | 10x | 10 mg |
| RTI-336 | 5-10x | 10-20 mg |
| WIN 35,065-2 | 5-10x | 10-20 mg |
| 1-phenyl-cocaine | 10x | 10 mg |
| 2-hydroxycocaine | 10x | 10 mg |
| 2β-ethoxycarbonylcocaine | est. 5x | 20 mg |
| 1-ethyl-cocaine | 3.5x | 28.57 mg |
| RTI-113 | 1.5x | 66.67 mg |
| 2β-Chlorococaine | est. 1.5x | 66.67 mg |
* The potency of HD/WF-23 isn't clear. The authors of the paper estimate 900 - 1200x, but the mathematics on the data for its IC50 stats comes out at 5400x (!!!!). Either way, it's brutal.
The Chemistry View: Tropane Nomenclature
| Name | Compound Name | Formula |
|---|---|---|
| WF-23-2 | 2β-Propanoyl-3β-(2-naphthyl)-tropane | C22H27NO2 |
| WF-23-1 | 2β-Propanoyl-3β-(2-naphthyl)-tropane | C22H27NO2 |
| RTI-55 | 2β-Carbomethoxy-3β-(4-iodophenyl)tropane | C20H24INO3 |
| RT1-121 | 2β-Carboisopropoxy-3β-(4-iodophenyl)tropane | C21H26INO3 |
| RTI-31 | 2β-Carbomethoxy-3β-(4'-chlorophenyl)tropane | C17H20ClNO4 |
| WF-24 | 2β-Ethyl ester-3β-(2-naphthyl)-tropane | C23H29NO2 |
| RTI-51 | 2β-Carbomethoxy-3β-(4-bromophenyl)tropane | C16H22BrNO2 |
| RTI-32 | 2β-Carbomethoxy-3β-(4-tolyl)tropane | C18H23NO2 |
| WF-30 | 2β-Propanoyl-3β-(1-naphthyl)-tropane | C22H25NO2 |
| WF-11/2-PTT | 2β-Ethyl ester-3β-(para-methylphenyl)-tropane | C21H27NO2 |
| RTI-112 | 2β-carbomethoxy-3β-(3-methyl-4-chlorophenyl)tropane | C20H24ClNO2 |
| WIN-35428 | 2-β-Carbomethoxy-3-β-(4-fluorophenyl)tropane | C17H21NO4 |
| RTI-177 | 2β-(3-phenylisoxazol-5-yl)-3β-(4-chlorophenyl)tropane | C22H24ClN2O2 |
| RTI-336 | 2β-(3-(4-methylphenyl)isoxazol-5-yl)-3β-(4-chlorophenyl)tropane | C23H26ClN2O2 |
| WIN 35,065-2 | 2β-Carbomethoxy-3β-phenyltropane | C16H21NO4 |
| 1-phenyl-cocaine | 1-Phenyl-2β-carbomethoxy-3β-benzoyloxytropane | C17H21NO4 |
| 2-hydroxycocaine (Singh 185d) | 3β-Benzoyloxy-2′-hydroxytropane | C16H21NO4 |
| 2β-ethoxycarbonylcocaine (Singh 201c) | 2β-Ethoxycarbonyl-3β-benzoyloxytropane | C18H23NO4 |
| 1-ethyl-cocaine | 1-Ethyl-2β-carbomethoxy-3β-benzoyloxytropane | C18H23NO4 |
| RTI-113 | 2β-carbophenoxy-3β-(4-chlorophenyl)tropane | C21H22ClNO2 |
| 2β-chlorococaine (Singh 201b) | 2β-Chloro-3β-benzoyloxytropane | C17H20ClNO4 |
The Chemistry View: IUPAC Nomenclature
| Name | Compound Name | Formula |
|---|---|---|
| WF-23-2 | 1-[(1S,3S,4R,5R)-8-methyl-3-naphthalen-2-yl-8-azabicyclo[3.2.1]octan-4-yl]propan-1-one | C22H27NO2 |
| WF-23-1 | 1-[(1S,3S,4R,5R)-8-methyl-3-naphthalen-2-yl-8-azabicyclo[3.2.1]octan-4-yl]propan-1-one (Variant 1) | C22H27NO2 |
| RTI-55 | Methyl (1R,2S,3S,5S)-3-(4-iodophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C20H24INO3 |
| RT1-121 | Isopropyl (1R,2S,3S,5S)-3-(4-iodophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C21H26INO3 |
| RTI-31 | Methyl (1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C17H20ClNO4 |
| WF-24 | Ethyl (1R,2S,3S,5S)-3-(naphthalen-2-yl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C23H29NO2 |
| RTI-51 | Methyl (1R,2S,3S,5S)-3-(4-bromophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C16H22BrNO2 |
| RTI-32 | Methyl (1R,2S,3S,5S)-3-(4-tolyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C18H23NO2 |
| WF-30 | 1-[(1S,3S,4R,5R)-8-methyl-3-(1-naphthyl)-8-azabicyclo[3.2.1]octan-4-yl]propan-1-one | C22H25NO2 |
| WF-11/2-PTT | Ethyl (1R,2S,3S,5S)-3-(4-methylphenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C21H27NO2 |
| RTI-112 | Methyl (1R,2S,3S,5S)-3-(3-methyl-4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C20H24ClNO2 |
| WIN-35428 | Methyl (1R,2S,3S,5S)-3-(4-fluorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C17H21NO4 |
| RTI-177 | (1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylic acid 3-phenylisoxazole | C22H24ClN2O2 |
| RTI-336 | (1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | |
| WIN 35,065-2 | Ethyl (1R,2S,3S,5S)-3-phenyl-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C16H21NO4 |
| 1-phenyl-cocaine | (1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylic acid phenyl ester | C17H21NO4 |
| 2-Hydroxycocaine | (1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]oct-2-en-2-ol | C16H21NO4 |
| 2β-Ethoxycarbonylcocaine | Ethyl (1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C18H23NO4 |
| 1-ethyl-cocaine | N-Ethyl-(1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate | C18H23NO4 |
| RTI-113 | Methyl (1R,2S,3S,5S)-3-(4-chlorophenyl)-8-azabicyclo[3.2.1]octane-2-carboxylate phenyl ester | C21H22ClNO2 |
| 2β-chlorococaine | (1R,2R,3S,5S)-2-chloro-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane | C17H20ClNO4 |
* Most of these are guessed values and your author was very tired.
The SMILES Reference
| Name | SMILES |
|---|---|
| WF-23-2 | CCC(=O)[C@H]1[C@H]2CCC@@HN2C |
| WF-23-1 | CCC(=O)[C@H]1[C@H]2CCC@@HN2C |
| RTI-55 | CN1[C@H]2CC[C@@H]1C@HC(=O)OC |
| RT1-121 | CN3[C@@H]1CCC3CC@@Hc2ccc(I)cc2 |
| RTI-31 | CN1[C@H]2CC[C@@H]1C@HC(=O)OC |
| WF-24* | CC(=O)O[C@H]1[C@H]2CCC@@HN2C |
| RTI-51 | Brc1ccc(cc1)[C@H]2C[C@@H]3N(C)C@HCC3 |
| RTI-32 | CC1=CC=C(C=C1)[C@H]2C[C@@H]3CCC@HN3C |
| WF-30 | CC(=O)O[C@H]1[C@H]2CCC@@HN2 |
| WF-11/2-PTT* | CC(=O)O[C@H]1[C@H]2CCC@@HN2C |
| RTI-112 | CC1=C(C=CC(=C1)[C@H]2C[C@@H]3CCC@HN3C)Cl |
| WIN-35428 | CN1[C@H]2CC[C@@H]1C@HC(=O)OC |
| RTI-177 | CN1[C@H]2CC[C@@H]1C@HC4=CC(=NO4)C5=CC=CC=C5 |
| RTI-336 | CC1=CC=C(C=C1)C2=NOC(=C2)[C@@H]3[C@H]4CCC@HC[C@@H]3C5=CC=C(C=C5) |
| WIN 35,065-2 | CN1[C@H]2CC[C@@H]1C@HC(=O)OC |
| 1-phenyl-cocaine* | CO2C[C@H]1[C@H]2CCC@@HC@HOC(=O)C4=CC=CC=C4 |
| 2-hydroxycocaine* | O[C@H]1[C@H]2CCC@@HN2C |
| 2β-ethoxycarbonylcocaine* | CCOC(=O)[C@H]1[C@H]2CCC@@HN(C)2 |
| 1-ethyl-cocaine* | CCN1[C@H]2CCC@@HC@HC(=O)OCH3 |
| RTI-113 | CN1C2CCC1C@HC(=O)OC4=CC=CC=C4 |
| 2β-chlorococaine* | Cl[C@H]1[C@H]2CCC@@HN2C |
* These are guessed values and your author was very tired.
See also: Monoamine Transporter Binding, Locomotor Activity, and Drug Discrimination Properties of 3-(4-Substituted-phenyl)tropane-2-carboxylic Acid Methyl Ester Isomers - F Ivy Carroll 1, Scott P Runyon, Philip Abraham, Hernan Navarro, Michael J Kuhar, Gerald T Pollard, James L Howard
https://pubmed.ncbi.nlm.nih.gov/15566309/
What we then say is:
- The RTI series are the Fentanyls of cocaine.
- WF-23 is the Carfentanil of cocaine; a single dose killed a lot of rats and the effects apparently lasted for days.
What Are We To Make Of WF-23 and RTI-55?
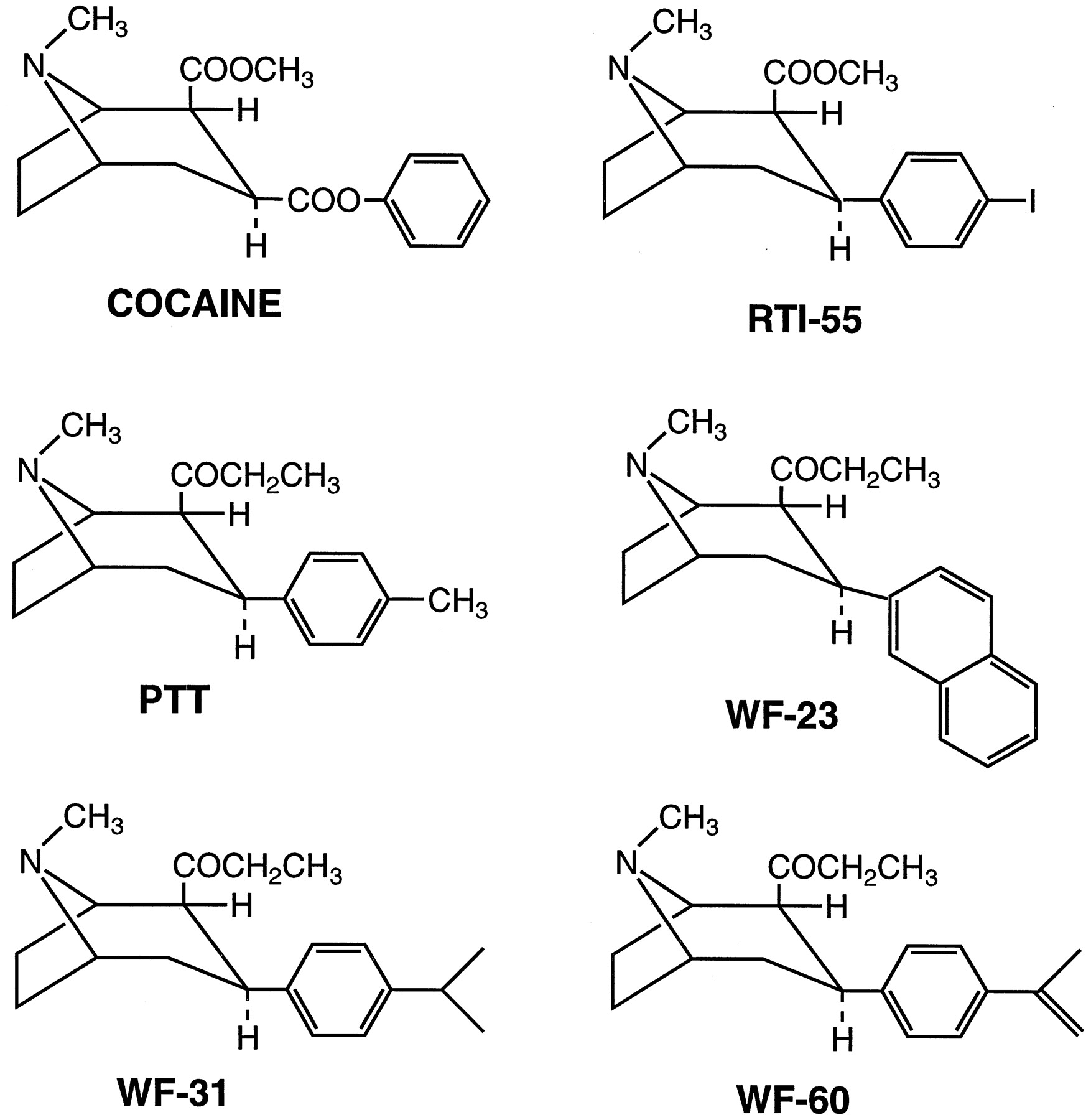
These two compounds are insane: the first is 1000x more potent than cocaine.
HD-23/WF-23
Let's take a closer look at what's going on here with 2β-Propanoyl-3β-(2-naphthyl)-tropane and why it is so potent, breaking it down the same way. Davies has increased the compound's complexity by swapping out the 2-beta-carbomethoxy for a propanoyl, and changed the 3-beta-benzoxy for a naphthyl. The result is two isomers with a 800-fold leap in potency.
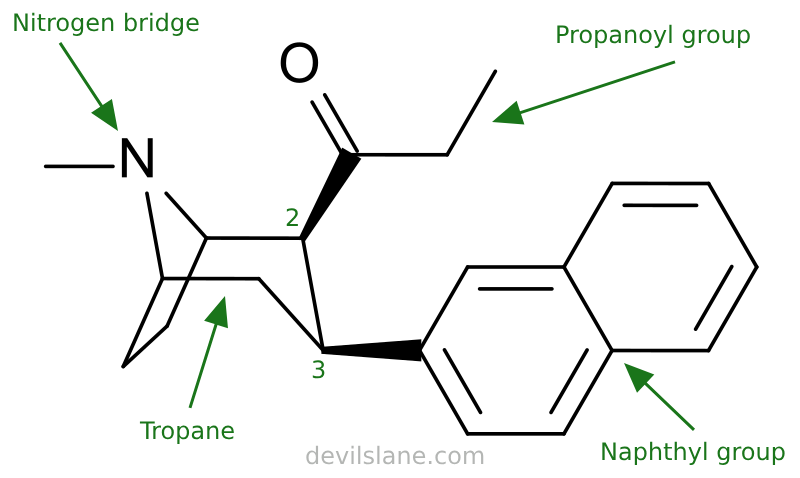
- 2-beta-propanoyl
- 3-beta-(2-naphthyl)
- tropane (the base molecule)
A 2-beta-propanoyl group consists of a propanoyl group (also known as a propionyl group) attached to the 2-beta position of the tropane base.
- "2" refers to the position of the substitution on the main ring system.
- "Beta" (β) indicates the stereochemical configuration at the 2-position, meaning the functional group is oriented above the plane of the main ring system.
- "Propanoyl" is derived from propanoic acid (CH₃CH₂COOH). It consists of a carbonyl group (C=O) bonded to an ethyl group (CH₃CH₂-).
A 3-beta-(2-naphthyl) consists of a 2-naphthyl group attached to the 3-beta position of the tropane.
- "3" refers to the position of the substitution on the main ring system.
- "Beta" (β) indicates the stereochemical configuration at the 3-position, meaning the functional group is oriented above the plane of the main ring system.
- "(2-naphthyl)" refers to a 2-naphthyl group, which is a naphthalene ring system connected at its 2-position to the main molecule. Naphthalene is a polycyclic aromatic hydrocarbon consisting of two fused benzene rings.
RTI-55 (Iometopane)
Let's also take a closer look at what's going on here with 2β-carbomethoxy-3β-(4-iodophenyl)tropane and why it is so potent, breaking it down similarly. Carroll has increased the compound's complexity by swapping out the benzoxy for a 4-iodophenyl group. The result is a compound with a 150-fold leap in potency.
RTI-55 is derived from Clarke's WIN 35,065-2 (Troparil). Its iodine atom means it affects serotonin a lot more than the others. This is related to its use in radiology, which requires radioiodine for single-photon emission computed tomography.
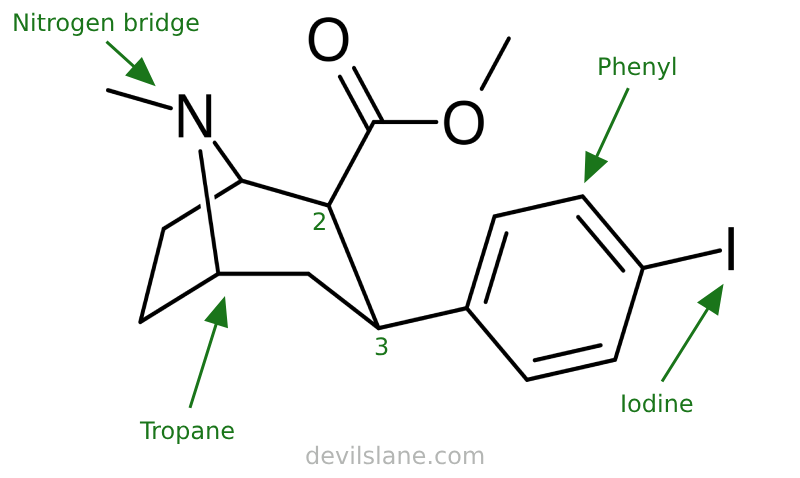
- 2-beta-carbomethoxy (ester group, same as cocaine)
- 3-beta-(4-iodophenyl)
- tropane (base molecule)
A 4-iodophenyl group consists of a phenyl ring (a benzene ring) with an iodine atom attached at the 4-position (also known as the para position).
- "Phenyl" refers to a benzene ring, which is a six-membered aromatic ring composed of carbon and hydrogen atoms (C₆H₅-).
- "4" or "para" refers to the position of the substitution on the phenyl ring. In this case, the iodine atom is attached to the carbon atom directly opposite to the point of attachment of the phenyl ring to the main molecule.
- "Iodo" indicates the presence of an iodine atom (I) as a substituent on the phenyl ring.
Here's the process:
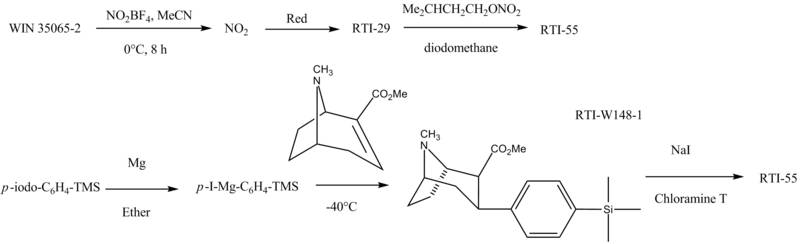
1970s: Tufariello, Clarke, & Lazer
J. J. Tufariello is a medicinal chemist who has made significant contributions to the field of drug discovery, particularly in the area of cocaine analogs and other stimulants.
Edward S. Lazer is a pharmacologist who has studied the effects of drugs of abuse on the brain and behavior. He has conducted research on the pharmacology and toxicology of various stimulants, including cocaine and amphetamines, and has contributed to the development of new treatments for addiction.
Robert Clarke is a neuroscientist who has studied the mechanisms of addiction and the development of new treatments for substance abuse disorders. He has conducted research on the effects of drugs of abuse on brain function and behavior, and has worked on the development of new pharmacotherapies for addiction.
Clarke created the "WIN" series of compounds at the Sterling WINthrop Institute in Northumberland. Compounds 13 and 24 became known as WIN-35428 and WIN 35,065-2.
Background Reading
- A synthesis of dl-cocaine using nitrone intermediates - J. J. Tufariello and G. B. Mullen
https://pubs.acs.org/doi/pdf/10.1021/ja00479a078 - Synthesis in the tropane class of alkaloids. Pseudotropine and dl-cocaine Joseph J. Tufariello, George B. Mullen, John J. Tegeler, Eugene J. Trybulski, Shing Chun Wong, and S. Asrof Ali
https://pubs.acs.org/doi/pdf/10.1021/ja00503a033 - Synthesis and biological activity of cocaine analogues. 2. 6H-[2]Benzopyrano[4,3-c]pyridin-6-ones - E S Lazer, G J Hite, K A Nieforth, E S Stratford
https://pubmed.ncbi.nlm.nih.gov/448684/ - Synthesis and Biological Activity of Cocaine Analogs I: N-Alkylated Norcocaine Derivatives - Edward S. Lazer, Naranjan D. Aggarwal, Gilbert J. Hite, Karl A. Nieforth, Roger T. Kelleher, Roger D. Spealman, Charles R. Schuster §, William Wolverton
https://www.sciencedirect.com/science/article/abs/pii/S0022354915423627 - Compounds Affecting the Central Nervous System. 4. 3.beta.-Phenyltropane-2-carboxylic Esters and Analogs - Robert Clarke, Sol Daum, Anthony Gambino, Mario Aceto, Jack Pearl, Morton Levitt, Wayne Cuminskey, and Eugenio Bogado
https://pubs.acs.org/doi/pdf/10.1021/jm00269a600
1980s & 1990s: Davies, Meltzer, Carroll & RTI
Peter C. Meltzer is a medicinal chemist who has made significant contributions to the field of drug discovery, particularly in the area of cocaine analogs and other stimulants.
F. Ivy Carroll is arguably the greatest expert in the world on cocaines - a distinguished medicinal chemist who has made significant contributions to the field of drug discovery, particularly in the area of cocaine analogs and other stimulants. He has been involved in the synthesis and testing of various compounds, with the goal of developing new treatments for addiction and other psychiatric disorders. Carroll has published over 500 papers in peer-reviewed journals and has been involved in numerous patent applications related to his research. He has received several awards for his contributions to the field, including the NAS Award for Chemistry in Service to Society.
https://gradschoolmagazine.unc.edu/2022/04/celebrated-chemist-frank-ivy-carroll-61-ph-d-committed-to-doing-something-good/
The Research Triangle Institute (RTI) is a renowned non-profit research organization based in North Carolina, with a staff of over 5,000 people and has been awarded numerous grants and contracts from government agencies and private foundations.
In its time, RTI has synthesised an enormous amount of compounds:
- RTI-31 : (–)-2β-Carbomethoxy-3β-(4'-chlorophenyl)tropane (64x)
- RTI-32 : (–)-2β-Carbomethoxy-3β-(4-tolyl)tropane (6x)
- RTI-51 : (–)-2β-Carbomethoxy-3β-(4-bromophenyl)tropane (3-10x)
- RTI-55 : (–)-2β-Carbomethoxy-3β-(4-iodophenyl)tropane (3-10x)
- RTI-83 : (–)-2β-carbomethoxy-3β-(4-ethylphenyl)tropane
- RTI-112 : 2β-carbomethoxy-3β-(3-methyl-4-chlorophenyl)tropane
- RTI-120 : (–)-2β-Carbophenoxy-3β-(p-tolyl)tropane
- RTI-121 : (–)-2β-Carboisopropoxy-3β-(4-iodophenyl)tropane (10x)
- RTI-126 : (–)-2β-(1,2,4-oxadiazol-5-methyl)-3β-phenyltropane (5x)
- RTI-150 : (−)-2β-Carbocyclobutoxy-3β-(4-methylphenyl)tropane (5x)
- RTI-160 : benzoylecgonine dimethylamide
- RTI-171 : (–)-2β-(3-Methylisoxazol-5-yl)-3β-(p-tolyl)tropane
- RTI-177 : 2β-(3-phenylisoxazol-5-yl)-3β-(4-chlorophenyl)tropane
- RTI-229 : (–)-3β-(4-iodophenyl)tropane-2β-pyrrolidine carboxamide
- RTI-274 : 2β-((3,4-Methylenedioxyphenoxy)methyl)-3α-(4-fluorophenyl)nortropane
- RTI-336: (−)-2β-(3-(4-methylphenyl)isoxazol-5-yl)-3β-(4-chlorophenyl)tropane
methyl (1R,2S,3S,5S)-3-(4-ethyl-3-iodophenyl)-8-azabicyclo[3.2.1]octane-2-carboxylate - RTI-371 : 3β-(4-Methylphenyl)-2β-[3-(4-chlorophenyl)isoxazol-5-yl]tropane
More: https://en.wikipedia.org/wiki/Category:RTI_compounds
Huw M.L. Davies is a distinguished chemist who has made significant contributions to the field of organic synthesis, particularly in the area of C-H functionalization and catalysis. He is currently a professor of chemistry at Emory University in Atlanta, Georgia, USA. Imagine being a student in this guy's class.
Huw, and the teams he was part of, created the "HD" ("Huw Davies", his name) and WF ("Wake Forest" University) series of cocaines. He takes the record by creating WF-23, the most powerful known synthetic cocaine analog.
https://www.sciencedirect.com/science/article/abs/pii/092241069390063F
The original HD series data is highly interesting (a lower IC50 value indicates higher potency):
- HD Analog 13 (P2-NT, 2-Naphthyl COCH2CH3 H): The most potent with an IC50 of 0.20 nM. This suggests it requires the least amount to inhibit a target by 50%.
- HD Analog 15 (Cyclohexyl COCH2CH3 H): Very potent with an IC50 of 4.610 nM.
- HD Analog 5 (PTT, Ph-pCH3 COCH2CH3 H): Also highly potent with an IC50 of 8.2 nM.
- HD Analog 4 (Ph-pCH3 COCH3 H): Shows high potency with an IC50 of 9.8 nM.
- HD Analog 12 (1-Naphthyl COCH3 H): Potent with an IC50 of 10.1 nM.
- HD Analog 2 (Ph COCH2CH3 H): Moderate potency with an IC50 of 47.3 nM.
- HD Analog 3 (Ph-pF COCH3 H): Moderate potency with an IC50 of 70.8 nM.
- HD Analog 1 (Ph COCH3 H): Moderate potency with an IC50 of 114 nM.
- (+)-WIN 35065 (Ph COOCH3 H): More potent than (-)-Cocaine with an IC50 of 98.8 nM.
In 1994 at Wake Forest university, Davies was also involved with the patented WF series of compounds related to his earlier work, which can be found here if you can't get the journal PDF: https://patents.google.com/patent/AU6767194A/en
- WF-4 (Ph-pCH3 COCH3 H): Very potent with a K_i of 9.8 nM.
- WF-8 (1-naphthyl COCH3 H): Very potent with a K_i of 10.1 nM.
- WF-11PTT (Ph-pCH3 COCH2CH3 H): Extremely potent with a K_i of 8.2 nM.
- WF-23 (2-naphthyl COCH2CH3 H): Exceptionally potent with a K_i of 0.20 nM.
- WF-23 Variant 2 (2-naphthyl COCH2CH3 H): Unprecedentedly potent with a K_i of 0.032 nM.
- WF-24 (2-naphthyl H COCH2CH3): Highly potent with a K_i of 2.51 nM.
- WF-30 (1-naphthyl COCH2CH3 H): Significantly potent with a K_i of 5.34 nM.
John F. Casale is a forensic chemist who has worked on the analysis and characterization of drugs of abuse, including cocaine and other stimulants. He has developed new methods for the detection and quantification of drugs in biological samples and has testified as an expert witness in several criminal cases related to drug trafficking and abuse. His work is instructive and clear, and a unique perspective from the law enforcement side of things.
Background Reading
- The Discovery of an Unusually Selective and Novel Cocaine Analog: Difluoropine. Synthesis and Inhibition of Binding at Cocaine Recognition Sites - Peter C. Meltzer, Anna. Y. Liang, and Bertha. K. Madras
https://pubs.acs.org/doi/10.1021/jm00039a014 - N-Modified analogs of cocaine: synthesis and inhibition of binding to the cocaine receptor - Philip Abraham, J. Bruce Pitner, Anita H. Lewin, John W. Boja, Michael J. Kuhar, and F. Ivy Carroll
https://pubs.acs.org/doi/pdf/10.1021/jm00079a018 - New, potent cocaine analogs: ligand binding and transport studies in rat striatum Author links open overlay panel - John W. Boja, F. Ivy Carroll, M. Abdur Rahman, Abraham Philip, Anita H. Lewin, Michael J. Kuhar
https://www.sciencedirect.com/science/article/abs/pii/001429999090627I - High Potency Cocaine Analogs: Neurochemical, Imaging, and Behavioral Studies - J. W. Boja, e. J. Cline, f. I. Carroll, a. H. Lewin, a. Philip, r. Dannals, d. Wong, u. Scheffel, m. J. Kuhar
https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.1992.tb25974.x - N-Modified fluorophenyltropane analogs of cocaine with high affinity for cocaine receptors - B.K. Madras, J.B. Kamien, M.A. Fahey, D.R. Canfield, R.A. Milius, J.K. Saha, J.L. Neumeyer, R.D. Spealman
https://www.sciencedirect.com/science/article/abs/pii/009130579090384T - 3-Aryl-2-(3'-substituted-1',2',4'-oxadiazol-5'-yl)tropane analogs of cocaine: affinities at the cocaine binding site at the dopamine, serotonin, and norepinephrine transporters - F. Ivy Carroll, Jeffrey L. Gray, Philip Abraham, Michael A. Kuzemko, Anita H. Lewin, John W. Boja, and Michael J. Kuhar
https://pubs.acs.org/doi/pdf/10.1021/jm00072a007 - 2.beta.-Substituted analogs of cocaine. Synthesis and inhibition of binding to the cocaine receptor - Anita H. Lewin, Yigong Gao, Philip Abraham, John W. Boja, Michael J. Kuhar, and F. Ivy Carroll
https://pubs.acs.org/doi/abs/10.1021/jm00079a017 - Novel 2-substituted cocaine analogs: binding properties at dopamine transport sites in rat striatum - Huw M.L. Davies, Elie Saikalia, Tammy Sextona, Steven R. Childersa
https://www.sciencedirect.com/science/article/abs/pii/092241069390063F - Cocaine and 3.beta.-(4'-Substituted phenyl)tropane-2.beta.-carboxylic Acid Ester and Amide Analogs. New High-Affinity and Selective Compounds for the Dopamine Transporter - F. Ivy Carroll, Pravin Kotian, Ali Dehghani, Jeffrey L. Gray, Michael A. Kuzemko, Karol A. Parham, Philip Abraham, Anita H. Lewin, John W. Boja, and Michael J. Kuhar
https://pubs.acs.org/doi/10.1021/jm00002a020 - Development of 3-Phenyltropane Analogues with High Affinity for the Dopamine and Serotonin Transporters and Low Affinity for the Norepinephrine Transporter - Chunyang Jin, Hernán A. Navarro, and F. Ivy Carroll
https://pubs.acs.org/doi/10.1021/jm801162z - A practical total synthesis of cocaine's enantiomers - John F. Casale
https://www.sciencedirect.com/science/article/abs/pii/0379073887901095 - Behavioral effects of novel cocaine analogs: a comparison with in vivo receptor binding potency - E J Cline, U Scheffel, J W Boja, F I Carroll, J L Katz, M J Kuhar
https://pubmed.ncbi.nlm.nih.gov/1545384/ - Structure-activity relationship of cocaine analogs: The synthesis of 3-beta-substituted cocaine analogs and computer-aided prediction of the pharmacological activity of cocaine analogs - Yang, Biao
https://archive.hshsl.umaryland.edu/handle/10713/1586
2000s: Singh, Kozikowski, Cheng
Satendra Singh is a pharmacologist who has studied the effects of drugs of abuse on the brain and behavior. His work on cocaine analogs is seminal to the field, like Clarke. His analogs aren't as potent, but his work is foundational in understanding the molecule's structure and relationships.
Singh notes modifications at the 3β-phenyl ring, demonstrate significant affinity for the DAT. For instance, compounds with electron-donating or withdrawing groups at the 4′-position of the phenyl ring, such as 4′-chloro, 4′-bromo, and 4′-iodo substituents, exhibit high binding affinities. And compounds derived from the structure of benztropine, are another class that shows high affinity and selectivity towards the DAT.
Particularly:
- Vinylogous 2β-position carbmethoxy-ester functional replacements. substitution with Cl (201b) or CO2Et (201c).
- 3β-(4′-Iodophenyl)tropane-2β-carboxylic acid isopropyl ester (25a) and 3β-(4′-Chlorophenyl)tropane-2β-carboxylic acid isopropyl ester (24a) are noted for their high potency and selectivity towards the DAT over 5-HTT and NET.
- 3β-(4′-Methylphenyl)tropane-2β-carboxylic acid phenyl ester (26f) and 3β-(4′-Chlorophenyl)tropane-2β-N-morpholino carboxamide (27l) are highlighted for their exceptional selectivity, indicating a promising direction for developing cocaine antagonists with minimal effects.
Alan P. Kozikowski is a medicinal chemist who has made significant contributions to the field of drug discovery, particularly in the area of neurological and psychiatric disorders. He has worked on the development of new drugs for the treatment of various conditions, including addiction, Alzheimer's disease, and schizophrenia. Kozikowski has published over 500 papers in peer-reviewed journals and has been involved in numerous patent applications related to his research. He has received several awards for his contributions to the field, including the ACS Award for Creative Invention.
Maarten E. A. Reith is a pharmacologist who has studied the mechanisms of action of drugs of abuse, including cocaine and other stimulants. He has conducted research on the molecular and cellular basis of addiction and has contributed to the development of new treatments for substance abuse disorders.
Guolin Cheng is a medicinal chemist who has worked on the development of new drugs for the treatment of addiction and other psychiatric disorders. He has been involved in the synthesis and testing of various compounds. His novel synthesis of cocaine is a great read.
Background Reading
- Chemistry, Design, and Structure−Activity Relationship of Cocaine Antagonists - Satendra Singh
https://pubs.acs.org/doi/full/10.1021/cr9700538 - Synthesis of novel spirocyclic cocaine analogs using the Suzuki coupling - Sukumar Sakamuri, Clifford George b, Judith Flippen-Anderson b, Alan P Kozikowski
https://www.sciencedirect.com/science/article/abs/pii/S0040403900001131?via%3Dihub - 2′-Substituted analogs of cocaine: synthesis and dopamine transporter binding potencies - Tarek F. El-Moselhy, Kwasi S. Avor, Garo P. Basmadjian
https://onlinelibrary.wiley.com/doi/abs/10.1002/1521-4184(200109)334%3A8/9<275%3A%3AAID-ARDP275>3.0.CO%3B2-B - The synthesis of tricyclic cocaine analogs via the 1,3-dipolar cycloaddition of oxidopyridinium betaines - Miles P. Smith, Clifford George, Alan P. Kozikowski
https://pure.johnshopkins.edu/en/publications/the-synthesis-of-tricyclic-cocaine-analogs-via-the-13-dipolar-cyc-2/fingerprints/ - Chemistry and pharmacology of the piperidine-based analogues of cocaine. Identification of potent DAT inhibitors lacking the tropane skeleton - A P Kozikowski, G L Araldi, J Boja, W M Meil, K M Johnson, J L Flippen-Anderson, C George, E Saiah
https://pubmed.ncbi.nlm.nih.gov/9599245/ - SAR Studies of Piperidine-Based Analogues of Cocaine. 4. Effect of N-Modification and Ester Replacement - Pavel A. Petukhov, Jianrong Zhang, Alan P. Kozikowski, Cheng Z. Wang, Yan Ping Ye, Kenneth M. Johnson, and Srihari R. Tella
https://pubs.acs.org/doi/abs/10.1021/jm0200153 - Novel C-1 Substituted Cocaine Analogs Unlike Cocaine or Benztropine - Maarten E. A. Reith, corresponding author Solav Ali, Audrey Hashim, Imran S. Sheikh, Naresh Theddu, Narendra V. Gaddiraju, Suneet Mehrotra, Kyle C. Schmitt, Thomas F. Murray, Henry Sershen, Ellen M. Unterwald, and Franklin A. Davis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3477221/ - Total Synthesis of (−)-Cocaine and (−)-Ferruginine - Guolin Cheng, Xinyan Wang, Rui Zhu, Changwei Shao, Jimin Xu, and Yuefei Hu
https://pubs.acs.org/doi/abs/10.1021/jo200069m
Will We See A Clandestine Market Emerge?
It's impossible to deny producing these compounds is tough. At the least, we're talking a 25-stage reaction cycle, with little to no information on time, yield, or toxicity. But we do know if it is complex, it will be an expensive product because of the manufacturing costs. Which means it will end up in wealthy circles as a luxury powder.
The EU's drug authority says:
Although various methods exist for the synthesis of cocaine, they are less economic than extraction of the natural product. Typical precursors include atropine, tropinone and carbomethoxytropinone, none of which is listed in Table 1 of the above-mentioned United Nations 1988 Convention [United Nations 1988 Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances].
https://www.emcdda.europa.eu/publications/drug-profiles/cocaine_en
This is foolish. Where there's a will - and cash - there's a way. It's not infeasible someone's already tried this. And it's not too much of a stretch to synthesise the precursors if necessary.
Is it complex? Yes. Is it doable? Yes, if you have the economic means: atropine is simple to get hold of. And what we've seen with Fentanyl is the production cost is worth it for malicious actors if the scaling factor is high. Fentanyl is 1000x the potency of morphine; EF-23 is 1000x the potency of cocaine. That means fewer trips; easier to smuggle; and vastly more profitable.
Let's take a look at the complexity of LSD synthesis, which is not trivial either. It starts with Ergot (a fungus which grows on wheat, which generates Ergotamine) and proceeds through a 10-ish-step reaction cycle to the Ethylamide. A typical dose is 50−200 μg (millionths of a gram).
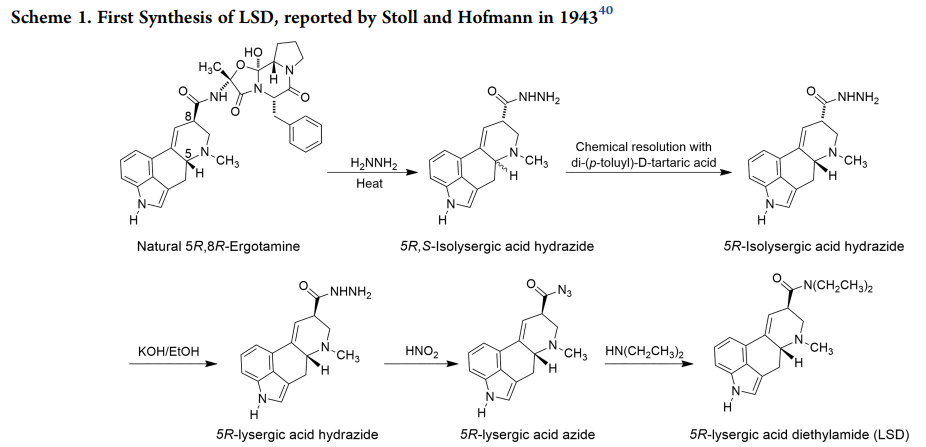
https://shaunlacob.com/wp-content/uploads/2020/12/DC-LSD.pdf
(Note: might do an article on this at some point as it's fascinating.)
But that said, there are two candidates who could make it happen:
- A state actor like China, who is already producing chemicals with the complexity of Fentanyl and shipping it to Mexico;
- An ultra-wealthy elite with the means to employ organic chemists to produce novel drugs to impress their rich friends.
The easiest way to do it would be to covertly redirect commercial supplies of Troparil and Iometopane, probably by skimming it from the factory or bribing a supplier.
What's interesting about a discussion like this is how it highlights the importance of agriculture in South America. It's perfectly possible for narco-gangs to build complex laboratories in remote places, with debt-stricken organic chemists who are bored. In fact, it's simpler in a lot of ways. It's simple to bribe chemical companies and steal medical supplies. The scaling differentials are much more favourable, and the scientific literature is there for anyone to scan - now with AI.
So the question is not "if", it is... when.
- A -
